
iMRS Prime Now Holds Health Canada Class II Approval
Canada regulatory update: This article discusses Canadian regulatory classification only. In the United States, iMRS Prime is presented as a wellness system and is not marketed for medical use.
We’re pleased to share that the iMRS Prime now holds a Health Canada Class II medical device licence, an important milestone for compliant availability in Canada.
This classification indicates that the iMRS Prime meets Health Canada requirements related to licensing for importation and distribution in Canada under the Class II framework. It supports compliant distribution and use within Canada for customers and organizations that require licensing under Canadian regulations.
What This Means (Canada)
-
The iMRS Prime is now registered as a Class II device in Canada.
-
It may now be made available to facilities that require regulatory authorization.
-
It strengthens product credibility and transparency within Canadian regulatory standards.
This aligns the iMRS Prime with other international regulatory recognitions and further positions it as one of the most internationally recognized PEMF systems available in North America.
A Milestone for the PEMF Community
For users, practitioners, and future owners of the iMRS Prime system, this milestone represents:
✔ Increased confidence in manufacturing and compliance
✔ Greater adoption potential in professional settings
✔ A positive step toward wider mainstream acceptance
While individual experiences with PEMF systems can vary, regulatory classifications like this can help create a clearer foundation for responsible distribution, education, and public understanding — particularly within Canada’s regulatory framework.
Health Canada Certificate
Below is the official Health Canada Class II certificate for the iMRS Prime:
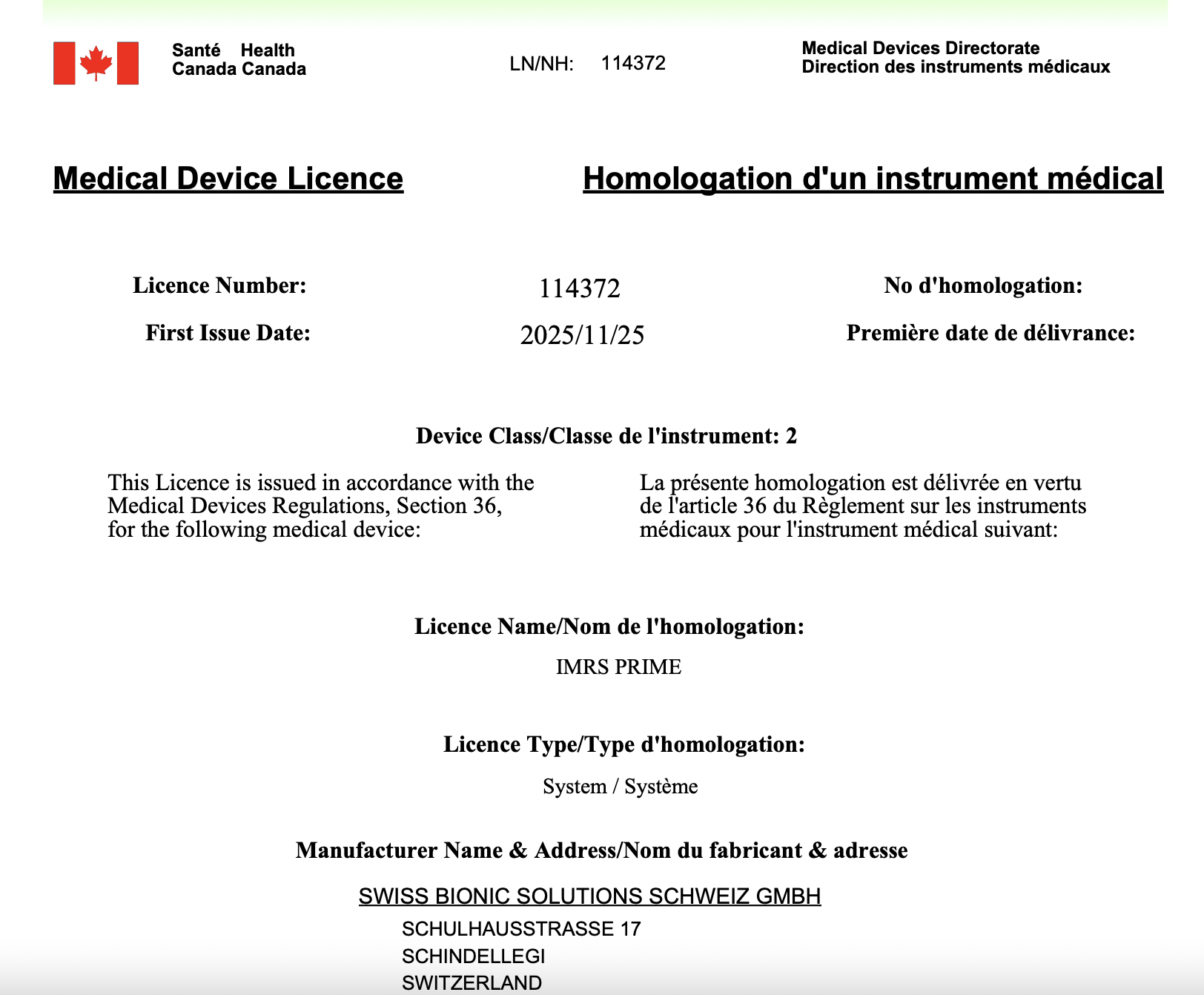
Official Health Canada Class II Certificate for the iMRS Prime PEMF System.
Health_Canada_Certificate_iMRS_prime
📌 Learn More About the iMRS Prime System
For a full overview of models, features, and technology architecture, you may visit our main iMRS Prime System Hub:
This announcement reflects Canadian regulatory classification for the iMRS Prime. It is provided for informational purposes only and does not imply therapeutic claims or usage guidance. Product availability, intended use, and marketing language vary by country and local regulatory standards.
